Catalytic reactions relevant to combustion and gasification
Start date: 2011-03-31
End date: 2018-12-31
The environmental consequences caused by fossil fuel combustion, such as production of pollutants and greenhouse gas, make the use of sustainable energy sources very attractive and important. Gasification and combustion, which convert carbonaceous material to a gaseous product with an employable heating value, are two of the most important and effective production methods of energy that enhances sustainable development.
Complex chemical reactions occur during gasification and combustion. In order to have a better understanding of the reactions mechanisms and how fast they occur, one needs to know the reactants, the products and how the products are formed from the reactants (i.e. one needs to know the intermediate structure called the transitions state). Quantum mechanics simulation tools are extensively used to provide detailed information of the chemical reactions. In this research, we use these tools to investigate some central reactions and the effect that catalysts have on these reaction mechanisms and rates.
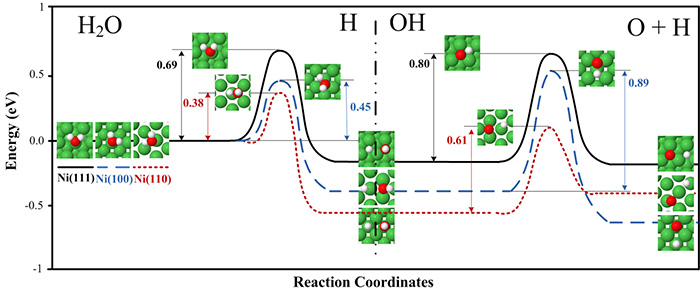
The results show that
-
The adsorption energies and dissociation rates depend on the surface structure.
-
The splitting of water to OH and H has lower activation energies over less packed Ni(110) and Ni(100) surfaces compared to the highly packed Ni(111) surface.
-
The subsequent splitting of the OH to O and H also has the lowest activation energy on the Ni(110) surface.
-
At 463 K, which is typical for industrial processes that include the water gas shift reaction, the H2O splitting is approximately 6000 and 10 times faster on the Ni(110) surface compared to the Ni(111) and Ni(100) surfaces, respectively, and OH splitting is 200 and 3000 times faster, respectively.
-
The complete water dissociation rate decreases in the order Ni(110) > Ni(100) > Ni(111).


